Understanding Monkeypox through the Lens of COVID
/We are living through two simultaneous pandemics.
Towards the end of 2019, we all took a crash course in virology and infectious disease epidemiology as we struggled with what was, for many of us, the first major pandemic of our lifetimes. We’ve learned a lot since then – we’ve upped our game when it comes to terms like basic reproduction number and lateral flow assay. And so, I think it might help to contextualize our second pandemic, the monkeypox pandemic, in the context of what we’ve learned about SARS-CoV-2.
Yes, we are going to compare apples to oranges. But in the end, fruit is fruit, and maybe by putting Monkeypox in a coronavirus context, we can be better prepared to face what is coming.
Let’s start with the basics.
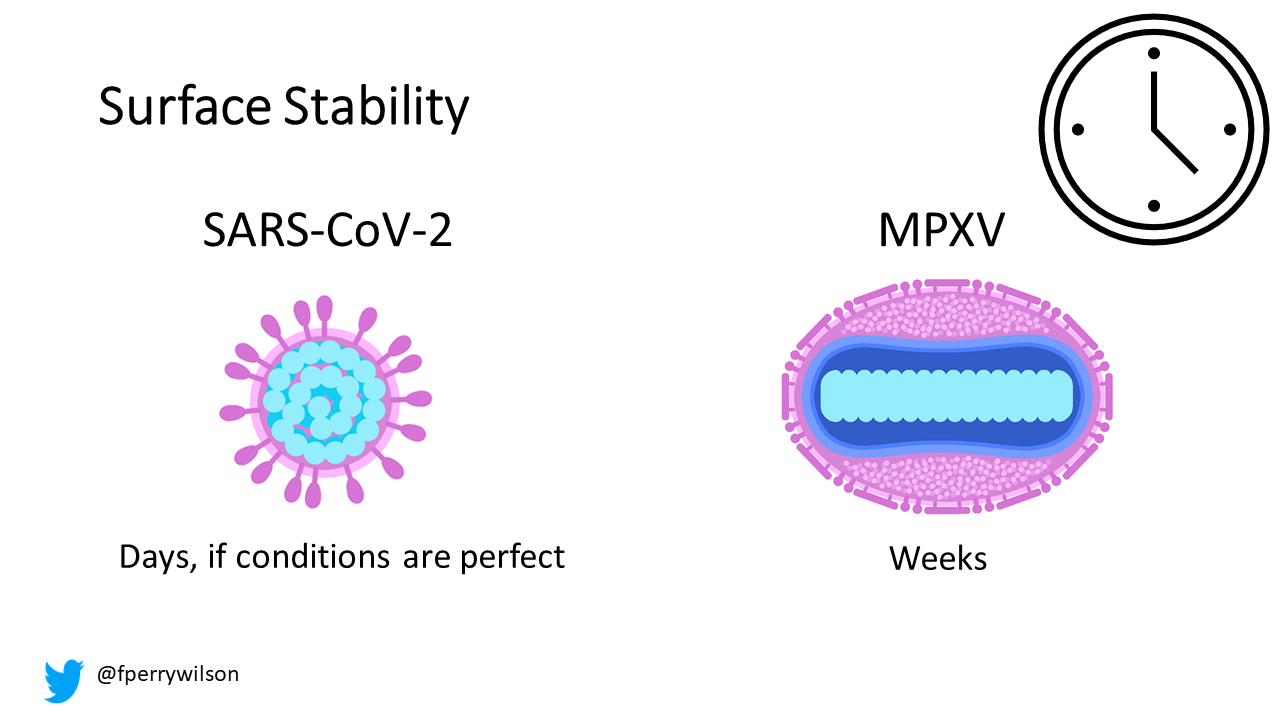
Here are the two current scourges we are facing. Note that SARS-CoV-2, the COVID virus, is a single-stranded RNA virus. It is small – with just 30 kilobases of nucleic acid inside. Monkeypox virus, like all pox viruses, is a big fella – a double-stranded DNA virus with nearly 200 kilobases of nuclear material.
This is an important difference. For one thing, RNA is much less stable than DNA. It breaks down if you look at it the wrong way. DNA is much heartier – and with a beefier viral envelope, it’s no surprise that monkeypox can hang out – alive - on surfaces for, according to the CDC, up to two weeks.
DNA is, generally, less subject to mutations than RNA. Data on the mutation rate of individual viruses are sort of all over the map, but to a first approximation, it seems like the mutation rate of SARS-CoV-2 is at least an order of magnitude higher than that of Monkeypox – perhaps 100-fold higher.
A caveat here – some data suggests a recent uptick in the monkeypox mutation rate. There are some interesting theories about why this may be, and how it may have led to the current pandemic. A bit out of scope for this, but suffice it to say that when it comes to our war against viruses, a rapid mutation rate is not in our favor.
One can’t discuss a virus without discussing the mode of transmission. SARS-CoV-2 is essentially airborne. It has a wild attack rate in enclosed spaces. Monkeypox, in part because of its larger size, is not transmitted as efficiently through this route (though it can be transmitted this way). Most of the current outbreak is due to skin-to-skin contact.
SARS-CoV-2 has what I’ve called before a “superpower” that let it take root around the world: the ability to transmit before symptoms occur. This is one major distinction between the current pandemic and the SARS-1 outbreak of 2002. In that outbreak, screening people for fever was an effective strategy to prevent its spread.
It remains unclear whether monkeypox can spread before symptoms develop. It’s clear it can spread before the rash develops, but I’m keeping a careful eye out for studies that suggest pre-symptomatic transmission. That would be very bad news.
Let’s talk about transmission. I hinted we’d get to the basic reproduction number (R0) which, as even my 7th-grader now knows, is the average number of individuals an infected person will infect in wild-type conditions – which is to say a fully susceptible population without mitigation strategies.
Estimates for the R0 of Omicron are crazy high – I see numbers like 10-15 thrown out pretty frequently, but these are built on a lot of assumptions about how bad things would be if most of us weren’t at least partially immune already. Suffice it to say, SARS-CoV-2 is a heck of a contagious virus.
Estimates of the R0 for Monkeypox, prior to the current outbreak tended to be south of 1, maybe as high as 1.2, which explains why so many small outbreaks don’t go anywhere – it just doesn’t spread to enough people. That inherent R0 has probably been climbing over time as population immunity declines due to the lack of smallpox vaccination. The WHO places the R0 for the current outbreak somewhere just south of two among communities of men who have sex with men – it’s probably lower than that in the general population, but we’re sort of too soon to tell. Regardless, it’s substantially lower than SARS-CoV-2. It may be high enough to sustain a pandemic, but the effect of a low-but-greater-than-1 R0 is to have a long, slow surge, rather than the dizzying spikes we’ve seen with the various coronavirus variants.
Both viruses can be pretty miserable to have – with fatigue and fever characterizing both syndromes. But SARS-CoV-2 is clearly more lethal, with something like a 0.5% mortality rate. The current outbreak gives monkeypox a mortality rate of 0.02% but, as COVID has taught us, deaths lag infections so that number could rise.
Interestingly the hospitalization rates for monkeypox and SARS-CoV-2 infections appear to be pretty similar at around 10%. But the reason for hospitalization differs. For SARS-CoV-2, individuals are typically hospitalized due to respiratory issues like hypoxemia. The primary driver of monkeypox hospitalization, so far, is the need for pain control.
I’ve often reflected on how “lucky” we were that SARS-CoV-2 was more severe in elderly individuals than in kids. That’s not a hard-and-fast viral rule. The CDC reports that children under the age of 8 may be at risk for more severe outcomes from Monkeypox due to a less developed immune system. That is certainly concerning.
Both viruses, at this point, have vaccines that can at least mitigate the worst outcomes, though the best monkeypox vaccine, Jynneos, is in very short supply. Both viruses have effective pill-based regimens. But the “paxlovid for monkeypox”, tecovirimat, is not technically FDA approved for this disease. Though tested on animals with monkeypox, it was approved to treat smallpox – may we never have to use it for that.
There we go – a completely apples-to-oranges comparison of two very different viruses. Nevertheless, I hope this makes it clear that, to the extent that we are destined to live through two simultaneous pandemics, the character of those two pandemics will be quite different.
A version of this commentary first appeared on Medscape.com.