A “Bionic Pancreas” for Type 1 Diabetes?
/New algorithms are changing the landscape of diabetes care, but a “functional cure” remains elusive.
It was 100 years ago when Leonard Thompson, age 13, received a reprieve from a death sentence. Young master Thompson had Type 1 diabetes – a disease that was uniformly fatal within months of diagnosis. But he received a new treatment, insulin, from a canine pancreas. He would live 13 more years before dying at age 26 of pneumonia.
The history of type 1 diabetes since that time has been a battle on two fronts. First, the search for a cause of and cure for the disease. Second, the effort to make the administration of insulin safer, more reliable, and easier.
The past two decades have seen a technological revolution in Type 1 diabetes care, with continuous glucose monitors decreasing the need for painful finger sticks, and insulin pumps allowing for more precise titration of doses.
The dream, of course, has been to combine those two technologies – continuous glucose monitoring and insulin pumps – to create so-called “closed loop” systems – basically an artificial pancreas – that would obviate the need for any intervention on the part of the patient save the occasional refilling of an insulin reservoir.
We aren’t there yet – but we are closer than ever.
Closed-loop systems for insulin delivery, like the Tandem Control IQ system, are a marvel of technology, but they are not exactly hands free. Users need to dial in settings for their insulin usage, count carbohydrates at meals, and inform the system that they are about to eat those meals to allow the algorithm to administer an appropriate insulin dose.
The perceived complexity of these systems may be responsible for why there are substantial disparities in the prescription of closed-loop systems. Kids of lower socioeconomic status are dramatically less likely to receive these advanced technologies. Providers may feel patients with lower health literacy or social supports are not “ideal” for these technologies, even though they lead to demonstrably better outcomes.
That means that easier might be better. And a “bionic pancreas” – as reported in this article from the New England Journal this week – is exactly that.
Broadly, it’s another closed-loop system. The bionic pancreas integrates with a continuous glucose monitor and administers insulin when needed. But the algorithm appears to be a bit smarter than what we have in existing devices. For example, the patient does not need to provide any information about their usual insulin doses – just their body weight. They don’t need to count carbohydrates at meals – just to inform the device when they are eating, and whether the meal is the usual amount they eat, more, or less. The algorithm learns and adapts as it is used. Easy.
And, in this randomized trial, easy does it.
219 participants were randomized in a 2:1 ratio to the bionic pancreas or usual diabetes care, though it was required that control participants used a continuous glucose monitor.
Participants were as young as 6 years old up to 79 years old, majority white, and had a relatively high household income. The mean A1C was around 7.8% at baseline.
By the end of the study, the A1C was significantly improved in the bionic pancreas group, with a mean of 7.3% vs 7.7% in the usual care group.
This effect was most pronounced in those with higher A1Cs at baseline, as you can see here.
People randomized to the bionic pancreas also spent more time in the target glucose range of 70 – 180 mg/dL.
All in all – the technology that makes it easy to manage your blood sugar, well, made it easy to manage your blood sugar.
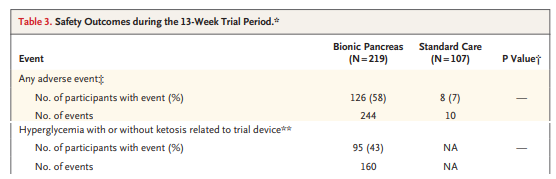
But new technology is never without its hiccups. You can see that those randomized to the bionic pancreas had a markedly higher rate of adverse events 244 events in 126 people compared to 10 events in 8 people in the usual care group.
This is actually a little misleading though – the vast majority of these events were hyperglycemic episodes due to infusion set failures – which were only reportable in the bionic pancreas group. In other words, the patients in the control group who had an infusion set failure (assuming they were using an insulin pump at all) would have just called their regular doctor to get things sorted and not reported it to the study team.
Nevertheless, these adverse events – not serious, but common – highlight the fact that good software is not the only key to solving the closed-loop problem. We need good hardware too – hardware that can withstand the very active lives children with Type 1 diabetes deserve to live.
In short, the dream of a functional cure to type 1 diabetes – a true artificial pancreas, is closer than ever, but it’s still just a dream. With iterative advancements like this though, the reality may be here before you know it.
A version of this commentary first appeared on Medscape.com.