In the Near Future, Your Life May Be Saved By a Robot
/New study demonstrates viability of a closed-loop shock-resuscitation system.
They call it the golden hour. Sixty minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires bloods, fluids, vasopressors – all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training – advanced life support (ALS).
If the patient is in a remote area, or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated… by a robot?
Enter “Resuscitation based on Functional Hemodynamic Monitoring” or “ReFit”, introduced in this article appearing in the journal Intensive Care Medicine Experimental.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field – blood, fluids, pressors. The researchers wanted to develop a closed-loop system. Something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device – an arterial catheter. The output – blood, fluids, and pressors, delivered intravenously.
The body of the system looks like this. It’s a prototype, obviously. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
If that’s the body, than this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
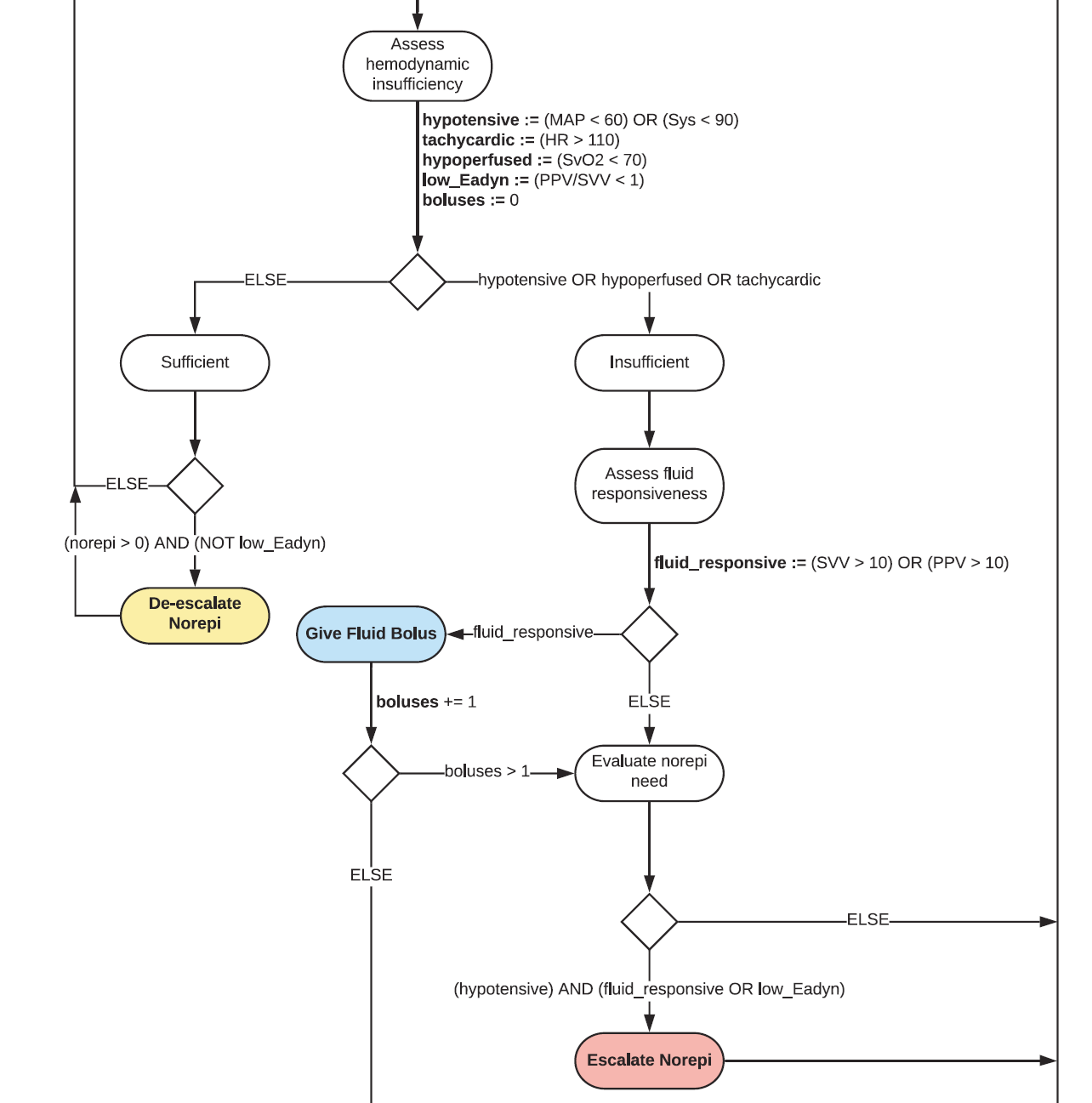
And If that’s the brain – than this is the mind – where the magic happens – the ReFit algorithm. The algorithm does its best to leverage all the data it can so I want to walk through it in a bit of detail.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
First, a check to see if the patient is stable or not – defined by a heart rate less than 110 and a mean arterial pressure greater than 60. If not, you’re off to the races – starting with a bolus of whole blood.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
And then the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors – in this case norepinephrine.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
And this cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team-members to do other things like getting the patient to a trauma center for definitive care.
So how do you test if something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances. In this case, pigs.
Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized, and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40mmHg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study.
Eight animals were plugged into the ReFit system.
For a window into what happens during this process – let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see the blood pressure start to fall precipitously after the liver lasceration. The heart rate quickly picks up to compensate – raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier.
The practical upshot of all of this is this… stabilization. Despite an as-of-yet untreated liver laceration.
Source: Pinsky et al. Intensive Care Medicine Experimental. 2024.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
Now this is all well and good in the lab – but in a real trauma, you actually need to transport a patient.
They tried this too. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny airport, and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war – and something that can extend the time you have to safely transport a patient to definitive care, well, that’s worth its weight in golden hours.
A version of this commentary first appeared on Medscape.com.