How Kids Can Be Affected by Arsenic Contamination
/A legacy of industry from nearly a century ago, arsenic is affecting our kids’ cardiovascular health today.
It’s easy to look at Onondaga lake, in upstate New York, and appreciate its placid beauty. Its clear cold waters are fed by a 285 square mile drainage basin, its meandering streams flow slowly into the Seneca river, eventually to Lake Ontario. It’s easy to look at a place like this and imagine that it looked similar hundreds or thousands of years ago, and it might have.
But it is not the same lake now as it was then. And what lies beneath the surface of the lake and the surrounding area may be hurting children.
The reason I am talking about this lovely lake is because of this paper, appearing in JAMA Network Open, examining the relationship between urinary arsenic levels and cardiovascular disease in children in the Syracuse area.
The study is an analysis of a larger cohort known as the Environmental Exposures and Child Health Outcomes cohort which is a cross-sectional study looking at the impact of various environmental toxins on children’s health.
The toxin of interest for today – arsenic.
Now you don’t need me to tell you that arsenic is toxic. Few poisons live as large in popular culture.
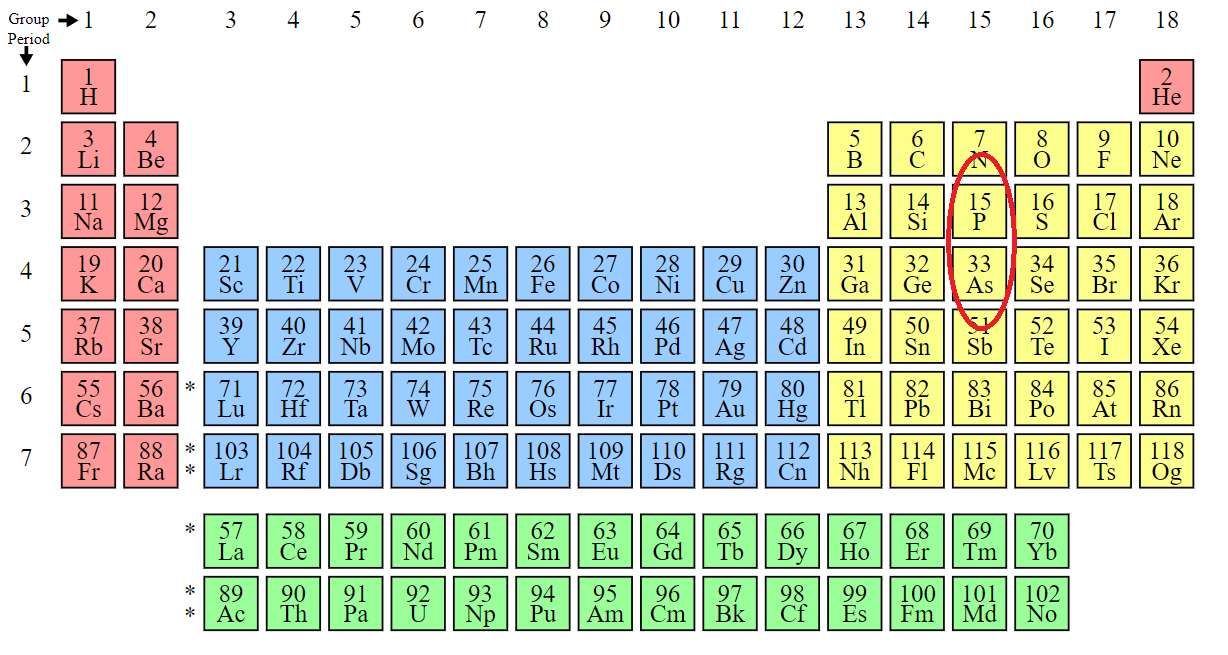
Living just below phosphorus on the periodic table, arsenic replaces phosphorus particularly in molecules containing certain sulfhydryl groups, leading to dysfunction of a slew of proteins and, with high enough doses, cell death.
But those effects are what you might see if you were dosed with arsenic in a glass of elderberry wine, for instance. Increasing amounts of data suggest that very low levels of arsenic also might have important, if less lethal, physiologic effects.
Multiple studies have documented that environmental arsenic exposure is associated with cardiovascular disease, likely through inhibition of nitric oxide synthase.
Studying its effects in children is hard, though, because, fortunately, kids don’t get much cardiovascular disease. The key insight in the study design we are discussing today, by Brooks Gump and colleagues at Syracuse University, was to use proxies of cardiovascular disease that can be detected in children: carotid artery intima-media thickness, left-ventricular hypertrophy, and carotid-femoral pulse-wave velocity. All of these measures have been shown to predict cardiovascular disease later in life. Let’s go to the results.
Two-hundred and forty-five children were enrolled and had arsenic levels measured in a single urine sample, with concentration adjusted for urinary creatinine level. The mean arsenic content in the urine was 7.76 micrograms per gram of creatinine – which is a bit higher than national averages in this age group. The range was wide though – with differences between the highest and lowest concentrations of roughly 500-fold.
As hypothesized, there were associations between those urinary arsenic levels and cardiovascular outcomes. This scatter plot shows the relationship between urinary arsenic and carotid intima-media thickness.
And here you can see the markedly higher urinary arsenic levels among kids with concentric left ventricular hypertrophy.
Where was this arsenic coming from? We can get arsenic from a variety of sources – most commonly drinking water contamination – and in many forms – with arsenic bound to organic molecules being less toxic than inorganic arsenic.
All the kids in this study got their drinking water from the same source – Skaneateles Lake – which has frequently been tested and has very little arsenic, so that’s not the culprit. Second-hand smoke is another source of arsenic, but exposure to smoking was not significantly associated with urinary arsenic in this cohort.
Dietary patterns did predict urinary arsenic levels – with fish and vegetable consumption appearing protective and fruit consumption leading to increased levels. But more interesting to me was their geographic clustering analysis. Did kids with high arsenic levels tend to live in the same area?
The yellow dots represent those clusters of kids with high arsenic levels in their urine, living southeast of Onondaga lake. That area is currently a superfund site due to more than a century of industrial pollution including – notably – heavy metals. The area around the lake was rich in limestone and salt, prompting Honeywell’s predecessor companies including the Solvay process company, pictured here, to build industrial facilities along the western shore.
Industrial activities there also included Allied Chemical’s mercury cell process, which discharged mercury and other heavy metals into the lake and surrounding areas.
And here we are, a century later, with the factories shuttered and the lake appearing clean and fresh as creation, and yet what lies beneath the surface is still there affecting our kids. A reminder that our choices today will echo.
A version of this commentary first appeared on Medscape.com.